Which of the Following Elements Will Produce the Same Spectrum
If the spectrum of a star is red or blue shifted then it can be used to infer its velocity along the line of sight. A continuous spectrum with the peak giving the temperature of the filament.
4 2 Understanding Atomic Spectra Chemistry Libretexts
Visible light is the smallest band in the electromagnetic spectrum true or false.

. No two elements produce the same spectrum. N 4 to n 3. 1 Describe the characteristics of each air mass.
Every atom gives discontinuous line spectra. Once the electron is excited to a higher energy level it quickly loses the energy and relaxes back to a more stable lower energy level. Which of the following elements behaves the most like the team pulling the hardest in the Figure C tug-of-war game.
The neon gas produces an emission line spectrum and most of the spectral lines are red orange and yellow. The spacing between the lines in the spectrum of an element are constant. N 2 to n 1.
Each element has a unique emission spectra that will be the same each. Correct option is C Explanation. These spectra are from the same element.
Each element in the periodic table can appear in gaseous form and produce its own spectrum unique to that element. Which is an emission spectrum which an absorption spectrum. An absorption spectrum with dark lines due to the solid filament.
N 3 to n 2. Astrophysicists can identify what kinds of materials are present in stars from the analysis of stars spectra. N 4 to n 2.
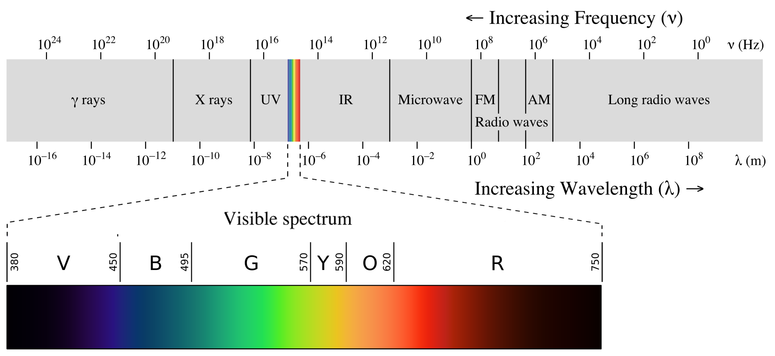
The visible spectrum showing the wavelengths corresponding to each color is shown below. Each line in the spectra corresponds to a specific wavelength and it is unique to a given element so no two elements give same pattern of lines in their spectra. When iron fuses into heavier elements it produces energy.
Either could be emission or absorption depending on the conditions with which they were made. What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition n 4 to n 2 of H e spectrum. Evidence of similar chemical properties is shown by elements present in the same ____ of the periodic table.
A continuum with bright tungsten lines added. Hydrogen will not look like Helium which will not look like carbon which will not look like iron. An incandescent light glowing tungsten filament produces.
Correct option is C A spectrum is an assembly of energy levels in the form of radiations emitted by an atom in its excited state. The following table shows a rough guide for the relationship between the temperature of a star and the electromagnetic spectrum. Log in for more information.
According to the question. An emission spectrum with bright lines due to ionized tungsten. Cant tell without knowing the element.
If the energy released is the same amount as the energy that makes up visible light the element produces a color. Rank the following from least to greatest in terms of the mass of the star that produces each. Carbon and oxygenhydrogen and heliumgold and silverNo two elements produce the same spectrum.
This is called the emission spectrum of an element. Which transition will produce the spectrum line with the lowest wavelength in this elements atomic spectrum. So the atoms becaome excited by absorbing energy like from a hot flame so this energy makes an electron or is it all electrons in the outer shell or is it all the shells move to a higher energy level and when the electron s return to its their ground state they give off energy in the.
Why is the Emission Spectrum the same every time.

Http Blade Rabbit Tumblr Com Post 142414288261 Salison There Is No Right Or Wrong In Color And Color Color Theory Design

Why Choose Led Grow Lights Led Lights Are The Best Choice Because They Produce Less Heat Use Less Electricity La Grow Lights Led Grow Lights Plant Lighting

Rgb Vs Cmyk Gdsiri Rgb Cmyk Color Additive Color Color Activities Color Theory
No comments for "Which of the Following Elements Will Produce the Same Spectrum"
Post a Comment